
CELLULAR BIOCHEMISTRY LAB @ TAMUK
APOPTOSIS
WHAT IS APOPTOSIS?
Programmed Cell Death is the type of cell death that occurs naturally as part of the normal development and tissue homeostasis of organisms. Most programmed cell death occurs by a morphologically defined process known as apoptosis. Cells undergoing apoptosis show characteristic morphological and biochemical features. The remains of apoptotic cells are rapidly phagocytosed by neighbouring cells or phagocytes without any leakage of the intracellular content into the extracellular space. This type of cell death, therefore, does not elicit an immune response which makes it perfectly suitable for its physiological role: the elimination of cells that have already served their purpose or damaged cells that could potentially harm the organism.
Apoptosis is observed during embryogenesis, metamorphosis, development of the nervous system, endocrine-dependent tissue atrophy, normal cell turnover, development of the immune repertoire and tumor regression. It is also becoming more evident that malfunctioning of the apoptotic process is involved in the outcome of many pathological disorders such as tumor progression, AIDS, cardiac arrest, autoimmune diseases and neurodegenerative diseases.
Apoptosis is a process regulated at the genetic level. bcl-2 was the first mammalian gene involved in apoptosis. The Bcl-2 protein has been shown to protect cells from cell death. Other genes have been discovered whose protein products protect from apoptosis. One such protein is Bcl-xL. Bcl-xL has also been shown to be a very powerful inhibitor of apoptosis. Our research interest focuses on the study of the mechanism by which these proteins protect from apoptosis, as well as the way these proteins are regulated. With this purpose, a new protein was isolated that binds to both Bcl-2 and Bcl-xL proteins (named BBP-1). Our efforts are dedicated to the biochemical and functional characterization of this protein as well as to the search of other proteins whose study may shed light into the mechanism of action and regulation of the activities of Bcl-2, Bcl-xL and BBP-1. The results obtained from these studies should increase the understanding of the apoptotic process at the molecular level. A better understanding of normal apoptosis will facilitate the finding of those factors that malfunction causing aberrant apoptosis that contributes to disease. In the long term, the availability of new information should help in the design and development of new therapeutic approaches to treat those human disorders in which apoptosis plays a role.
These are our publications in this field:
-
Cuende E, Alés-Martínez JE, Ding L, González-García M, Martínez-Alonso C and Núñez G: Programmed cell death by bcl-2-dependent and independent mechanisms in B lymphoma cells. EMBO Journal 1993, 12: 1555-1560.
-
Boise LH , González-García M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Núñez G and Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993, 74: 597-608.
-
Cleveland JL, Troppmair J, Packham G, Askew DS, Lloyd P, González-García M, Núñez G, Ihle JN and Rapp UR. v-raf supresses apoptosis and promotes growth of interleukin 3-dependent myeloid cells. Oncogene 1994, 9: 2217-2226.
-
González-García M, Pérez-Ballestero R, Ding L, Duan L, Boise LH, Thompson CB and Núñez G: bcl-xL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development 1994, 120: 3033-3042.
-
Núñez G, Merino R, Grillot D and González-García M. Bcl-2 and Bcl-x: regulatory switches for lymphoid death and survival. Immunol. Today 1994, 15 (12): 582-588.
-
González-García M, García I, Ding L, O'Shea S, Boise LH, Thompson CB and Núñez G. bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc. Natl. Acad. Sci. USA 1995, 92: 4304-4308.
-
Clarke MF, Apel IJ, Benedict MA, Eipers PG, Sumantran V, González-García M, Doedens M, Fukunaga N, Davidson B, Dick JE, Minn AJ, Boise LH, Thompson CB, Wicha M and Núñez G. A recombinant bcl-x s adenovirus selectively induces apoptosis in cancer cells, but not in normal bone marrow cells. Proc. Natl. Acad. Sci. USA 1996, 92: 11024-11028.
-
Grillot DAM, González-García M, Duan L, Inohara N and Núñez G. The murine bcl-x gene: genomic organization, promoter region and chromosome localization. J. Immunol. 1997, 158: 4750-4757.
-
Del Peso L, González-García M, Page C and Núñez G. Interleukin-3-Induced Phosphorylation of Proapoptotic BAD through the Protein Kinase Akt. Science 1997, 278: 687-689.
-
Chintarlapalli SR, Jasti M, Malladi S, Parsa KV, Ballestero RP, González-García M. BMRP is a Bcl-2 binding protein that induces apoptosis. J. Cell Biochem. 2005, 94: 611-626.
-
Malladi S., Parsa K.V., Bhupathi D., Rodriguez-Gonzalez M.A., Conde J.A. Anumula P., Romo H.E., Claunch C.J., Ballestero R.P., and González-García M. (2011) Deletion Mutational Analysis of BMRP, a Pro-Apoptotic Protein that Binds to Bcl-2. Mol Cell Biochem. 351:217-232.
-
Conde J.A., Claunch C.J, Romo H.E., Benito-Martín A., Ballestero R.P., and González-García M. (2012) Identification of a Motif in BMRP Required for Interaction with Bcl-2 by Site-directed Mutagenesis Studies. J. Cell Biochem 113: 3498-3508.
-
Wyatt S, Glover K, Dasanna S, Lewison M, González-García M, Colbert CL, Sinha SC. Epstein-Barr Virus Encoded BCL2, BHRF1, Downregulates Autophagy by Noncanonical Binding of BECN1. Biochemistry. 2023 Oct 17;62(20):2934-2951. doi: 10.1021/acs.biochem.3c00225. Epub 2023 Sep 30. PMID: 37776275; PMCID: PMC11166532.
Do you want to know a little bit more about apoptosis....?
What is APOPTOSIS? Apoptosis is the way by which "normal cell death" occurs. Our cells die because: they have already served their function, they are not longer needed, they are damaged and, if alive, they could be potentially harmful to the organism. Many cells die "normally" during development and tissue homeostasis. Normal cell death occurs by a morphologically defined process known as APOPTOSIS. Apoptotic cells shrink, their chromatin condenses, their intracellular organelles remain intact as well as their plasma membranes. Apoptotic cells end up fragmenting into smaller pieces known as apoptotic bodies. Apoptotic bodies are phagocytosed by macrophages or neighboring cells. At the center of acute lesions or injuries, however, cells die by a process known as NECROSIS . During necrosis, cells swell and finally burst which results in the leakage of the contents of the cell to the extracellular space. This elicits an inflammatory response which does not occur when cells die by apoptosis.
Malfunctioning of the apoptotic process contributes to human disease. The process of apoptosis has been involved in tumor development. To keep a constant number of cells in tissues, there has to be a balance between new cell formation (or proliferation) and cell death (apoptosis). If this balance is disrupted, either by an increase in proliferation or a decrease in apoptosis, a tumor develops. If the proliferation rate is normal but the rate of apoptotic cell death is increased, the opposite outcome is observed: loss of cells. This happens in diseases such as neurodegenerative diseases (Alzheimer's, Huntington's, Parkinson's,...).
Figure 1. Effect of bcl-2 and bcl-xL Expression on FL5.12 Survival following IL-3 Withdrawal. Apoptosis is a process controlled at the genetic level. There are some genes, such as bcl-2 and bcl-x , whose protein products protect cells from apoptosis. This figure shows that both Bcl-2 and Bcl-xL proteins protect the cell line FL5.12 from apoptosis induced by IL-3 (Interleukin-3) withdrawal from the growth media. FL5.12 is a proB lymphoid cell line that requires the presence of IL-3 in the growth media both for survival and proliferation. Stable transfectants of FL5.12 vector containing bcl-xL in the forward (closed square) and reverse (open square) orientations, bcl-2 (closed triangle), bcl-2+bcl-xL (closed diamond) and vector control (Neo; closed circle) were established. Cell survival was determined by trypan blue exclusion at the indicated time points. Data are presented as the mean +/- SD of triplicate cultures.

Figure 2. Apoptotic DNA Ladder of FL5.12 Transfected Cells. When cells die by apoptosis, their chromatin condenses and finally breaks down into DNA fragments that are multiple of 180 bp. Different transfectants were grown in RPMI-1640 supplemented with 10% FCS and 10% WEHI-3B(D)-conditional medium as a source of IL-3. At time 0, cells were washed several times with RPMI to deprive them from IL-3. At the different times showed, samples of 2 millions cells were harvested and total DNA extracted. 1 µg of total DNA was loaded on a 0.8% agarose gel. Lines: (1) FL5.12/Neo negative control transfectant, (2) FL5.12/bcl-2 , (3) FL5.12/bcl-xs , (4) FL5.12/bcl-2+ bcl-xs, (5) FL5.12/ bcl-xL , (M) Marker: 23.1, 9.4, 6.6, 4.3, 2.3, 2.0 and .56 Kb (l DNA/ Hin d III).
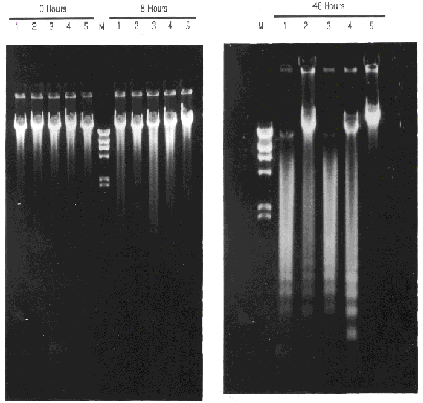
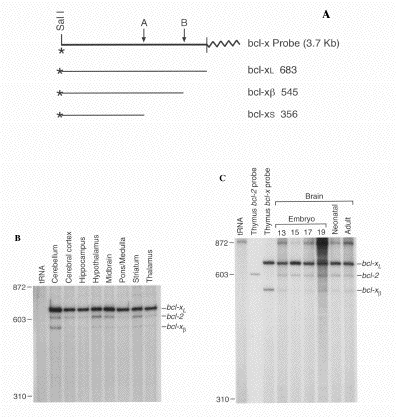
Figure 3. Expression of bcl2 and bcl-x mRNAs in Embryonic and Adult Neuronal Tissues of Mice. (A) S1 nuclease probe constructed to detect bcl-x mRNA forms. The region corresponding to the 5' splice donor site used in the generation of bcl-xs mRNA in the human is indicated by the A arrow. The exon/intron boundary in the bcl-xL cDNA is indicated by the B arrow. pBluescript sequences are indicated by a wavy line. Expected sizes of protected fragments for bcl-xL, bcl-xs and bcl-xß mRNA forms are indicated in nucleotides. (B and C) Equal amounts of end-labeled bcl-2 and bcl-x S1 nuclease probes were simultaneously hybridized to 10 µg of total RNA from adult tissues (B) or 5 µg of RNA from 13- to 19-day embryonic and neonatal tissues. (C) Hybridization of both probes with a control tRNA sample is shown for comparison. Hybridization of thymus mRNA to the bcl-2 or bcl-x probe alone is also shown. The bcl-2 probe protected a fragment of 600 nucleotides.